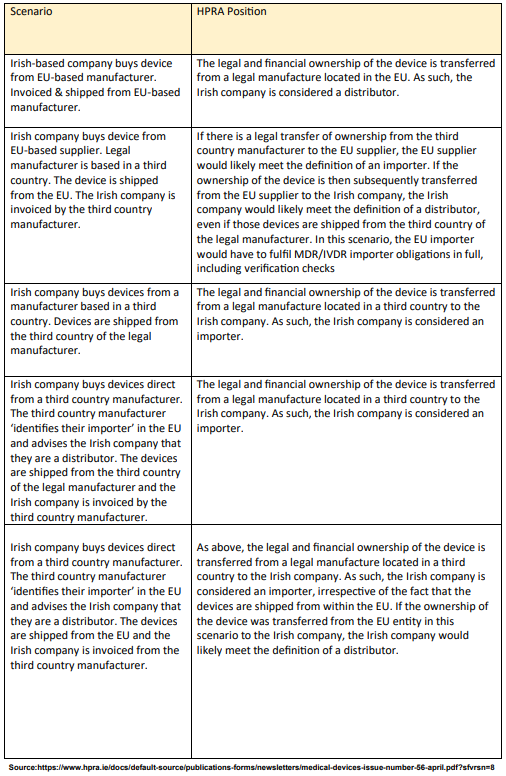
Understanding what distributors need to do under EU MDR and IVDR is a complicated topic. First, let’s make sure we are on the same page about what a distributor is. This is important because distributors and importers share many responsibilities and sometimes do similar things. However, their jobs and thus the MDR/IVDR Regulations they must follow can vary.
According to MDR, a distributor is any person or company in the supply chain that gets a medical device ready for sale. This does not include the manufacturers or importers. And it is also important to notice the responsibility is to get the product ready for sale but not for actual use. The key difference between a distributor and an importer is where they are in the supply chain:
- An importer is the one who introduces medical devices to the EU market for the very first time.
- Distributors are the ones who make these devices available on the market again (sometimes multiple times) until they reach the end user.
If an importer sells a medical device directly to the end users, they are still considered an importer, not a distributor. This is done to help reduce confusion. It also helps maintain transparency and honesty within the market and industry as a whole.
What requirements are needed from distributors before they can have a new product placed on the market?
Before a distributor can sell a device, they must follow certain steps to ensure its safety and verify where it comes from. Here’s what distributors need to do to be compliant with MDR/IVDR Regulations:
- Check that the product has been properly designated and carries the necessary EU marking.
- Confirm that there is an up-to-date and complete declaration of conformity issued.
- Make sure the device includes everything the manufacturer and suppliers require for it.
- Ensure that the importer’s information is on the product, packaging, or necessary paperwork.
- Double-check that the manufacturer has given the unique device identifier (UDI) as required.
- Verify that the product appears to meet or exceed the EU regulations and requirements.
To meet these requirements, distributors need to use a sampling method that accurately represents the devices they are selling. Moreover, while the distributor has the device, they must also ensure it is stored and transported according to the manufacturer’s specified conditions.
What requirements are needed from importers before they are allowed to send a new product to market?
The specific requirements for importers to place a new product on the market can vary depending on the country and the type of product. A general list of MDR/IVDR Regulations requirements can include:
- Ensure that the product complies with all relevant safety and technical standards.
- Conduct product testing and certification as required by regulatory authorities.
- Prepare accurate product documentation, including user manuals, labeling, and technical specifications.
- Maintain records of product testing, certification, and compliance documents.
- Obtain any necessary import licenses or permits from the relevant government authorities.
- Complete customs documentation, including import declarations and customs clearance forms.
To meet these requirements, importers will need to test and check the products and goods they receive thoroughly before any imported items can be allowed to go to market. Moreover, while the importer will be responsible to ensure proper documentations, payments, licenses, and other legal matters are taken care of during products handling and transfer.
What are the documentation and reporting requirements for distributors under MDR and IVDR?
A distributor’s responsibilities don’t end once they’ve made a device available on the market. In fact, there are a number of MDR/IVDR Regulations for documentation and reporting that distributors must follow carefully.
These include:
Keeping a detailed report of complaints. Distributors have to keep a record of complaints from healthcare professionals and patients. These reports also must be immediately forward that information to the manufacturer. In many cases, the authorized representative and importer must also be notified.
Informing officials of any nonconformances. The EU regulations give instructions for what needs to happen when distributors believe a device is not in conformance with regulations. They are required to inform all involved personnel as soon as possible about the issues and quality concerns.
Reporting serious incidents or serious risks. Distributors must be ready to provide all of their documentation that has been recorded about the product in question. They are also required to demonstrate the conformity of any device called into question and present proof of initial inspections.
Achieving a high level of traceability of devices. The regulations also state that distributors must be able to identify for competent authorities:
- Every economic operator they supplied a device to
- Every economic operator that supplied them with a device
- Every healthcare professional or institution they supplied
If all of these responsibilities are fulfilled, it should result in the quick tracing of any nonconforming devices and timely corrective actions that keep patients and providers safe.
Import your CE marked medical devices in Europe. Guarantee MDR and IVDR compliance while maintaining control, avoid channel complications and an efficient operation. GrowthImports is your dedicated independent import partner for all your European needs.
