Under the old medical device directives (MDD 93/42/EEC), the requirement for a distributor is not mentioned, while the word MDR importer appears three times. The new MDR (2017/745) implemented per May 26th 2021, revisits the entire supply chain and economic operators, namely: the manufacturer, authorized representative, importers, and distributors. Each economic operator will have replaced and additional responsibilities and liabilities.
This article will explain the potential risks and complications of designating your distributor as your importer and the benefits of having one independent EU wide importer.
What is the definition of an MDR Importer?
According to the Blue Guide / MDR the definition of the Importer is “Any natural or legal person established within the Union that places a device from a third country on the Union market”. A product is placed on the market when it is made available for the first time on the Union market.
A product is made available on the market when supplied for distribution, consumption or use on the Union market in the course of a commercial activity, whether in return for payment or free of charge.
Such supply includes any offer for distribution, consumption or use on the Union market which could result in actual supply (e.g. an invitation to purchase, advertising campaigns).
Supplying a product is only considered as making available on the Union market, when the product is intended for end use on the Union market. The supply of products whether for further distribution, for incorporation into a final product, or for further processing or refinement with the aim to export the final product outside the Union market is not considered as making available. Commercial activity is understood as providing goods in a business related context.
As for ‘making available’, the concept of placing on the market refers to each individual product, not to a type of product, and whether it was manufactured as an individual unit or in series. Consequently, even though a product model or type has been supplied before new Union harmonisation legislation laying down new mandatory requirements entered into force, individual units of the same model or type, which are placed on the market after the new requirements have become applicable, must comply with these new requirements.
Who can be the MDR Importer?
As explained previously an importer can be any natural or legal person established within the European Union. The reason for the MDR regulation to re-define and designate specific obligations to the Economic Operators, is to build additional checkpoints to avoid incompliance products to be placed in the Union. To be aligned with this philosophy and avoid any incompliance risks, it is advisable the Economic Operators are separate entities.
(1) Distributor
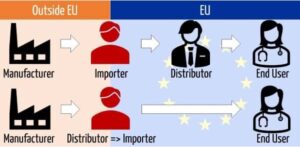
A distributor who directly purchases the goods from the manufacturer, clears it from customs and supplies it to the end-user can be appointed as the importer. Meaning that a distributor can be an importer. In Article 14 / MDR the obligations of the distributor are stated, and in this case the distributor also needs to comply with Article 13 which will make the distributor jointly liable for goods placed on the market. The complications of designating a distributor as the importer is described below.
(2) Multiple distributors / importers
A manufacturer can also appoint multiple distributors or importers. There is no limitation to the number of importers. However designating multiple importers also means more challenges and complications as described in the section below.
(3) Manufacturer
If the manufacturer has a subsidiary as an entity in the Union and supplies the goods direct to the end-user, the manufacturer can also act as the importer.
(4) Logistics / E-commerce
If goods are shipped to a 3PL /e-commerce company which will receive the goods directly from the manufacturer, stocks the goods and ships it out to a distributor or end-user, they will be seen as the MDR importer.
If you are using the services of a fulfilment provider and they are conducting activities such as storing products, packaging/relabelling, handling customer returns, etc., they might be considered a distributor by EU regulators.
(5) Independent importer
The importer can also be a separate entity / company designated by the manufacturer to fulfil the obligations of the MDR (article 13) importer requirements. At GrowthImports we fulfil this role by being specialised in the MDR importer role, providing European wide, independent hassle-free importing so manufacturers nor distributors do not have to struggle with the requirements and liabilities.
If you as a manufacturer want to sell direct into the EU market to a patient, healthcare professional or hospital, your end-user cannot be your importer. So if you are intending to sell direct into the market you would need to appoint an importer.
The risks and complications when appointing multiple importers or as distributor as the MDR importer
As explained above, the idea behind the MDR regulation is to build in more safety protocols and would recommend to designate separate entities within the Economic Operator supply chain to control and keep visibility on the traceability of goods. More-over it would facilitate additional compliance verifications and increased quality standards.
A manufacturer is however free to appoint a distributor as its importer. Complications and risks manufacturers in that case need to consider are:
(1) A quality contract that states the additional liabilities and obligations a distributor (article 14) will have and the addition of the importer obligations (article 13). It would mean that a distributor will be joint liable for the products placed on the market.
(2) Not all distributors have the capabilities nor the desire to take on these additional liabilities and obligations
(3) Imagine a distributor providing hundreds of SKU’s from different suppliers. It would require a distributor to have their own full regulatory department. Wouldn’t you as a manufacturer prefer your distributor keeps the focus on sales and marketing your products?
(4) If your distributor is also your importer your dependency in the market may be too much relying on your distributor. Not only if your distributor gets bankrupt, acquired but also when a distributor under performs, it may be challenging to leverage or influence the agreement or the complications when withdrawing from the relationship. Or imagine an incompliance of your importer that is also your distributor. The chances you will lose your business and reputation in the market will be substantial
(5) Sharing technical file documentations with your distributors could be sensitive and unlocking proprietary information
A manufacturer can also decide to designate multiple distributors. Also this construction could bring incompliance risks as well as market complications:
- The traceability of the devices needs to be tracked which can be challenging to map and include in the Post Market Surveillance. Moreover, each importer needs to add their label on the product which brings operational complexities
- At the end of the day the manufacturer will be liable of the supply chain and thus the quality and assurance of MDR compliance of the importer will need to be audited and verified by the manufacturer. With multiple importers this can bring challenges as well as increase compliance risks as multiple economic operators are involved
- If there are 2 distributors in a market and one distributor wants to be the importer and the other doesn’t, it means the label of the willing distributor will be added and shipped to all end-users. This could potentially create channel conflicts in the market
Why choose GrowthImports as your MDR compliant European wide importer?
GrowthImports is launched because of the MDR and need for manufacturers to work with an independent importer to avoid all the complications and compliance risks mentioned in previous sections.
Our clients not only have faced distributors or 3PL’s not wiling to take the importer obligations and joint-liabilities on them, but also chose for GrowthImports due to the convenience and accessibility of the processes we have designed to ensure hassle-free access to the European market while assuring compliance.
Many of our clients also do not want to burden the administrative tasks and operational complexities nor do they want to have their distributors lose focus or do they want to be too much dependent on their distributors. Moreover when developing new distribution partnerships and by already having an importer designated and registered, the chances to establish new partnership will be increased.
Overall partnering with one specialized, independent importer for all European wide importing needs will bring efficiency and guaranteed MDR compliance for a fraction of the cost.
Want to learn more about your specific situation and how we can help or would you like to learn about the experience of our cients? Book a call with us or contact us at info@growthimports.eu or feel free to visit our website www.growthimports.eu for more information/
