VAT on Medical Devices in the EU
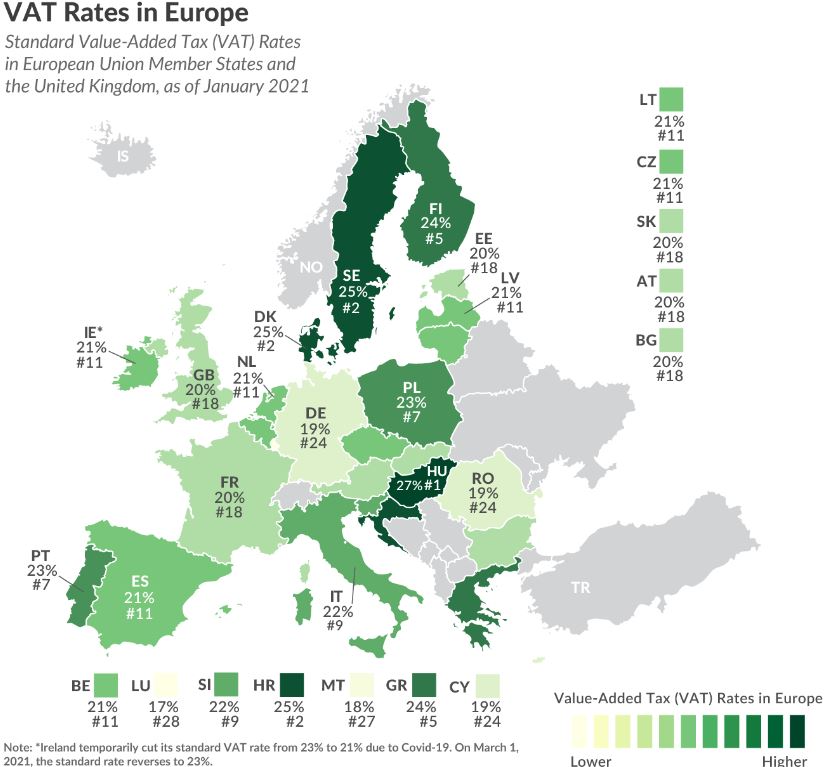
VAT in the EU can be complicated for some: While all VAT guidelines are set at the European Union level, member countries are allowed to
GrowthImports obtains ISO 13485:2016

GrowthImports obtains ISO 13485:2016. We’re very proud to announce we have been successfully audited for ISO 13485:2016. We are thankful for the great efforts of our team.
Welcoming Mrs. Elvan Inkaya
We are excited to announce Elvan Inkaya has joined GrowthImports as our new quality assurance director.
Whitepaper: Is your European medical device importer MDR Compliant?
Whitepaper: Is your European medical device importer MDR Compliant? According to Article 2 (33) of the MDR, the definition of the Importer is
MDR importers and distributor knowledge
The Medical Device Coordination Group (MDCG) has released questions related to importers and distributors under Regulation (EU) 2017/75
Book a meeting with us at Medica
GrowthImports will be attending the largest medical device tradeshow “Medica” November 15-18, 2021 at Dusseldorf Messe in Germany.
IVDR launch date potentially postponed

The In Vitro Diagnostic Medical Devices Regulation (EU) 2017/746 is scheduled to be implemented for May 26, 2022. Many European biotech
MDD/MDR importer during transition
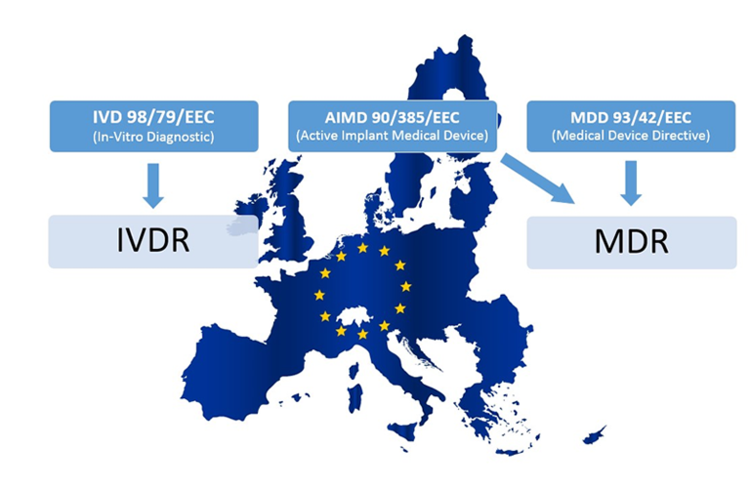
Transitioning MDD to MDR: Until May 2025, products certified under the Directives and products certified under the Regulations may coexist